Blog: Regulatory
Meeting ISBER Recommendations for Biobank Management
17th March 2020
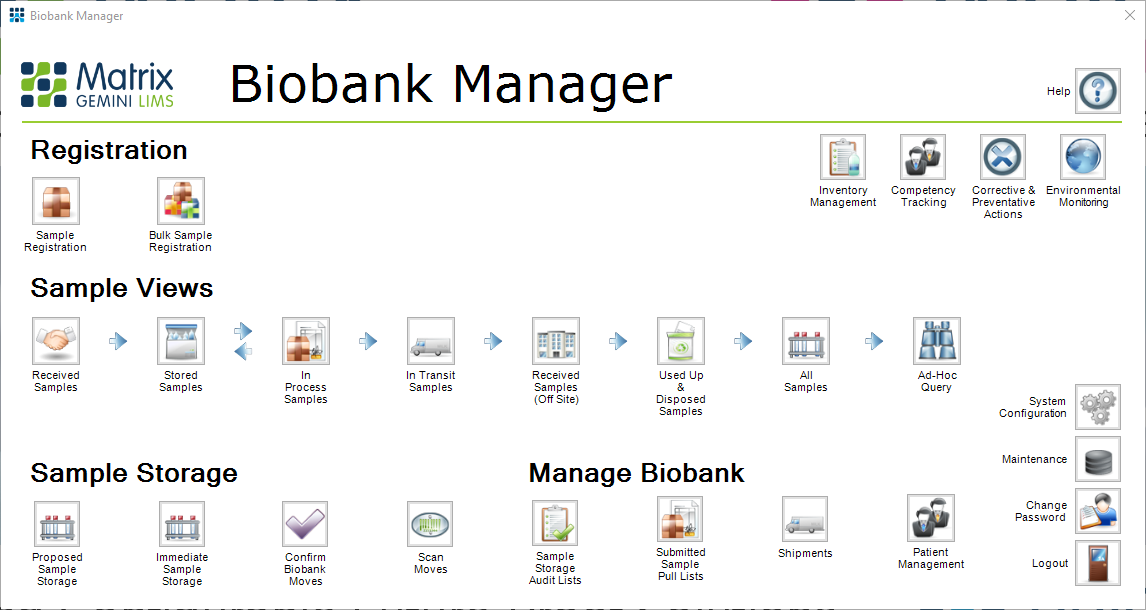
LIMS can contribute significantly to the efficient management of any biobank facility. With every aspect of specimen handling from collection through storage, processing, distribution, and eventual disposal to be considered as well as the need to manage all the associated data and information, ISBER (International Society for Biological and Environmental Repositories) has published a set of recommendations (the ISBER Best Practices: Recommendations for Repositories Fourth Edition) for the most effective practices for managing biological and environmental specimen collections and repositories. ISBER has also created a very useful self-assessment tool to allow biobanks to evaluate how well their facilities already comply with the best practices. In a recent webinar Autoscribe Informatics explained how the Xybion LIMS Biobank Manager configuration addresses the recommendations from ISBER. This webinar is still available to view.
Configurable Biobank Manager solution
Biobank Manager is a particular configuration of the Xybion LIMS. It is built using the Xybion LIMS configuration tools without the need for any coding. The webinar shows a demonstration of the standard Biobank Manager configuration and how it complies with the ISBER best practice requirements, but it can easily be modified using the configuration tools to reflect the look and feel and the precise workflows required by the individual biorepository. The starting point is the log-in screen which follows the ISBER requirements of having different levels of accesses for different user groups and everyone having an individual user code and password so that a complete audit trail of all actions can be created.
Storing samples
The first stage is to register samples received by the biobank into the LIMS. The types and quantities of samples received can be easily specified and allocated to a particular study. Sample and patient IDs can be entered manually or by scanning bar codes. Once samples have been registered, they have to be allocated to a particular storage location. The webinar goes on to show the flexibility in creating the database hierarchy for sample storage to reflect the actual physical storage. For example, it can be specified that a particular container type won’t fit into a particular rack, to prevent erroneous location allocation in the software. It is also possible to set up a proposed storage allocation for when some space has been created in a particular location. The proposed allocation can be confirmed to the actual allocation once the sample has been physically moved. Every step carried out is recorded in the database to provide a full chain of custody.
Removing and transferring samples
Samples may need to be transferred from one location to another within the biobank if they are needed in a different study. In addition, biobank samples may need to be removed from storage for testing or for transfer to another facility. Biobank Manager can easily handle all of these scenarios. A ‘pull’ list can be created, which identifies the samples required and their location. Once samples have been pulled for testing or external transfer, then full details of the destination and shipping information must be recorded so that the movement of the sample can be fully tracked. There is also a requirement to record the action on the sample at its new location. It may be transferred back after testing, or it may be consumed completely during testing, or the remainder may be disposed of after testing. Color coding is used extensively on the Biobank Manager screens to make it easy to identify the current status of any sample. Internal and external auditing can be carried out to confirm that any particular sample is in its specified location and to view the full chain of custody.
Ad-hoc queries
Another important requirement for ISBER Best Practice is the Ad-Hoc query. This screen within biobank Manager makes it possible to create queries to search for certain samples using a number of different filters and then display everything that has happened to these samples. Pull lists can then be created for any of these samples that fulfill the requirements for a further study.
And there’s more
There are many other modules that are integrated into Biobank Manager that feed into ISBER requirements such as the environmental monitoring module, instrument calibration and measurement module, competency tracking module and the corrective and preventative action module. These can all help with the efficient management of a biobank.
To find out more about how these additional modules support the ISBER recommendations and about Biobank Manager in general, call us on +44 118 984 1610/+1 508 457 7911. If you would like to watch the webinar, please click here.