
Autoscribe LIMS Blog



Applications
10 Steps to Ensure a Successful LIMS Implementation
Buying a LIMS can seem overwhelming given the choice of solutions on the market. This ten-step guide helps you through the process and arms you with the questions you need to ask.
18th July 2024

Business
What is the cost of a LIMS?
How much should you realistically expect to pay for a LIMS and what are the important factors to consider?
18th July 2024
Applications
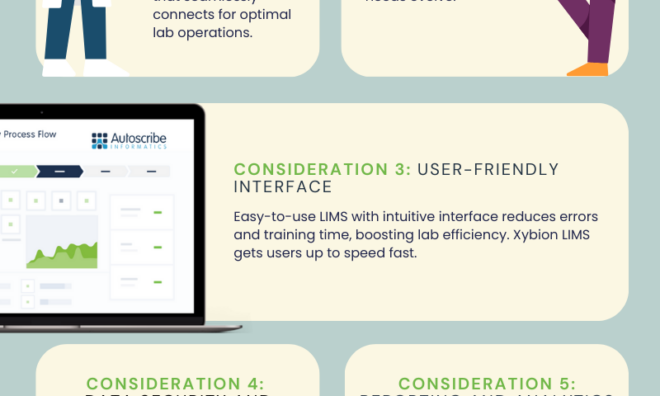
Evaluating a LIMS for Reagent and Lab Inventory Management: Seven Key Considerations
Are you considering a LIMS for reagent and lab inventory management? Make sure you consider these seven things when choosing a LIMS. Learn more here.
9th July 2024


Regulatory
FDA Continues to Highlight Data Integrity as a QC Lab Issue
FDA warning letters continue to highlight the issue of ensuring data integrity within laboratories. How are you ensuring data is not changed (deliberately or otherwise) and is stored securely? This article discusses the issue and one way of managing it to meet the stringent requirements of regulated laboratories.
10th June 2024
Let‘s Talk
Find out how we can help maximize efficiency and minimize risk for your laboratory.
Follow Autoscribe
Let’s Talk
Ready to get started? Contact us today.
Let’s connect and we’ll arrange a Xybion LIMS demo.